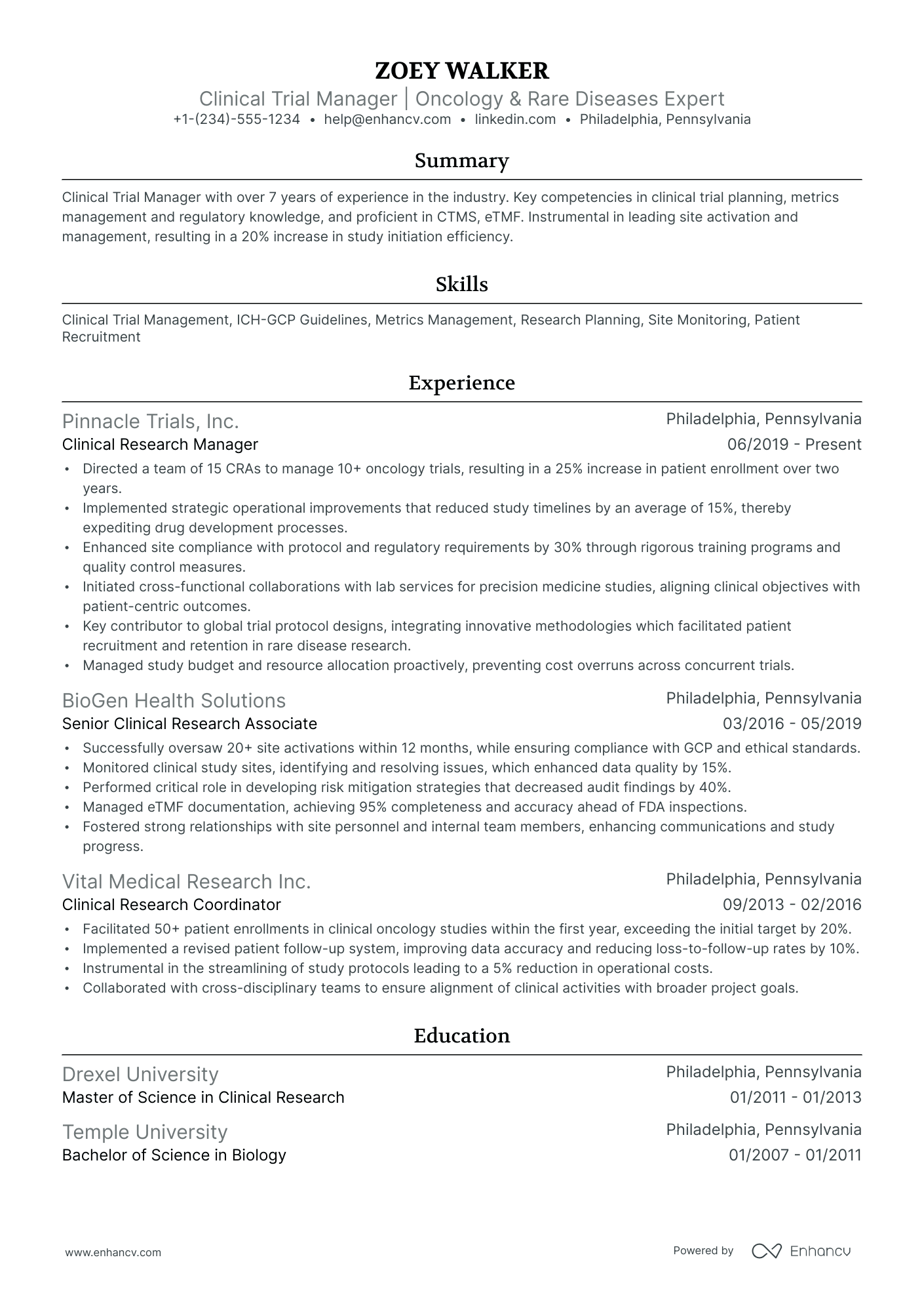
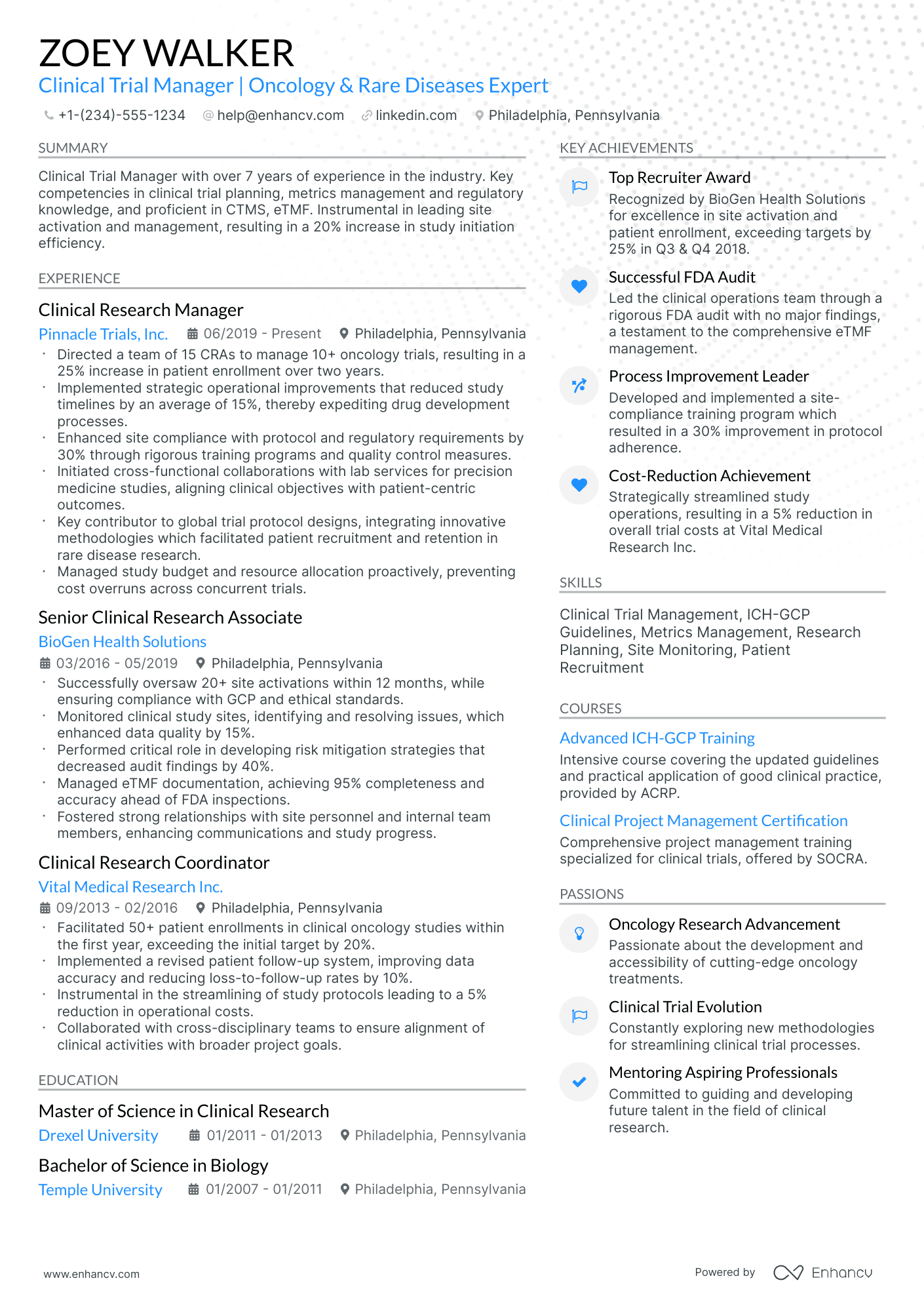
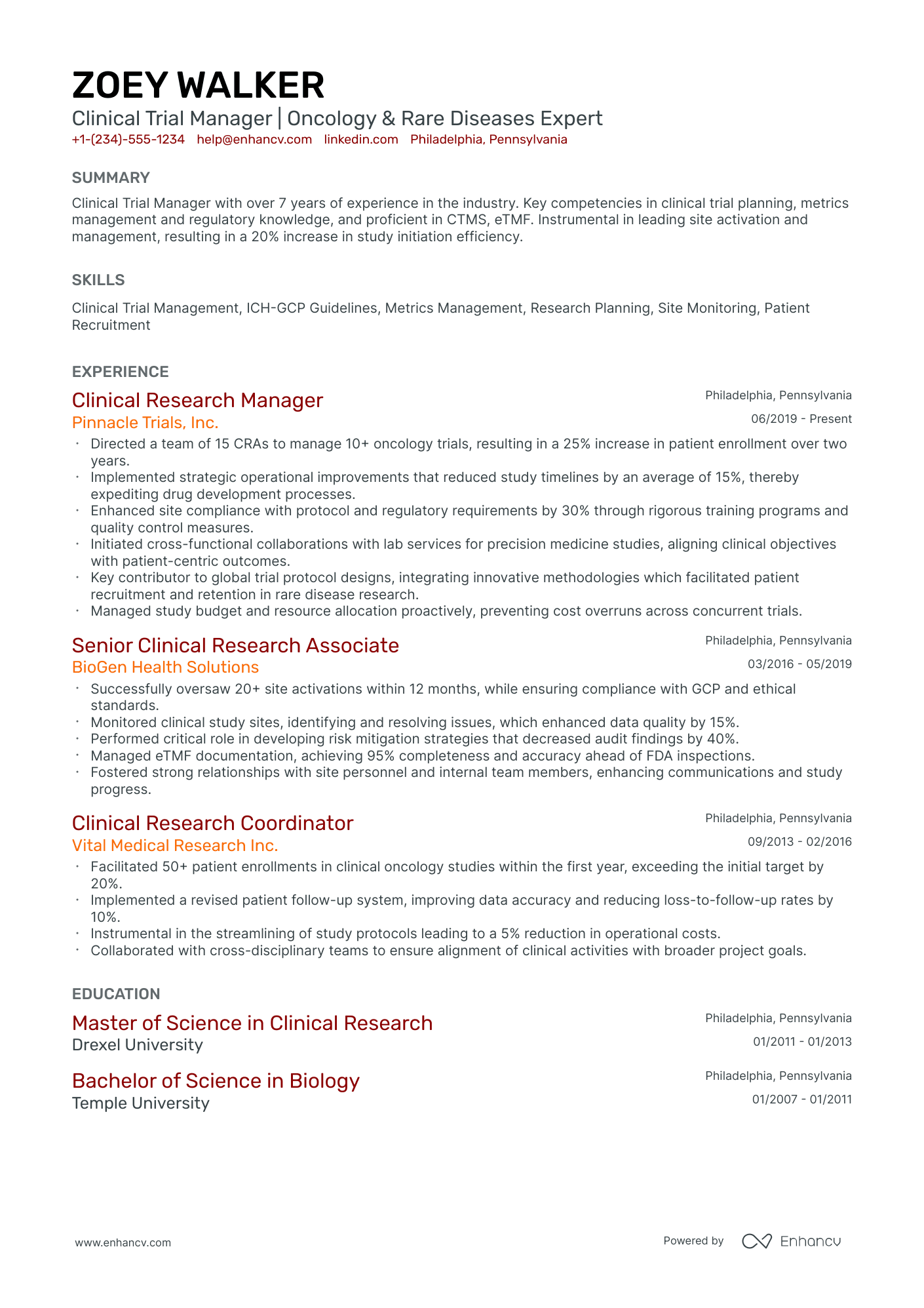
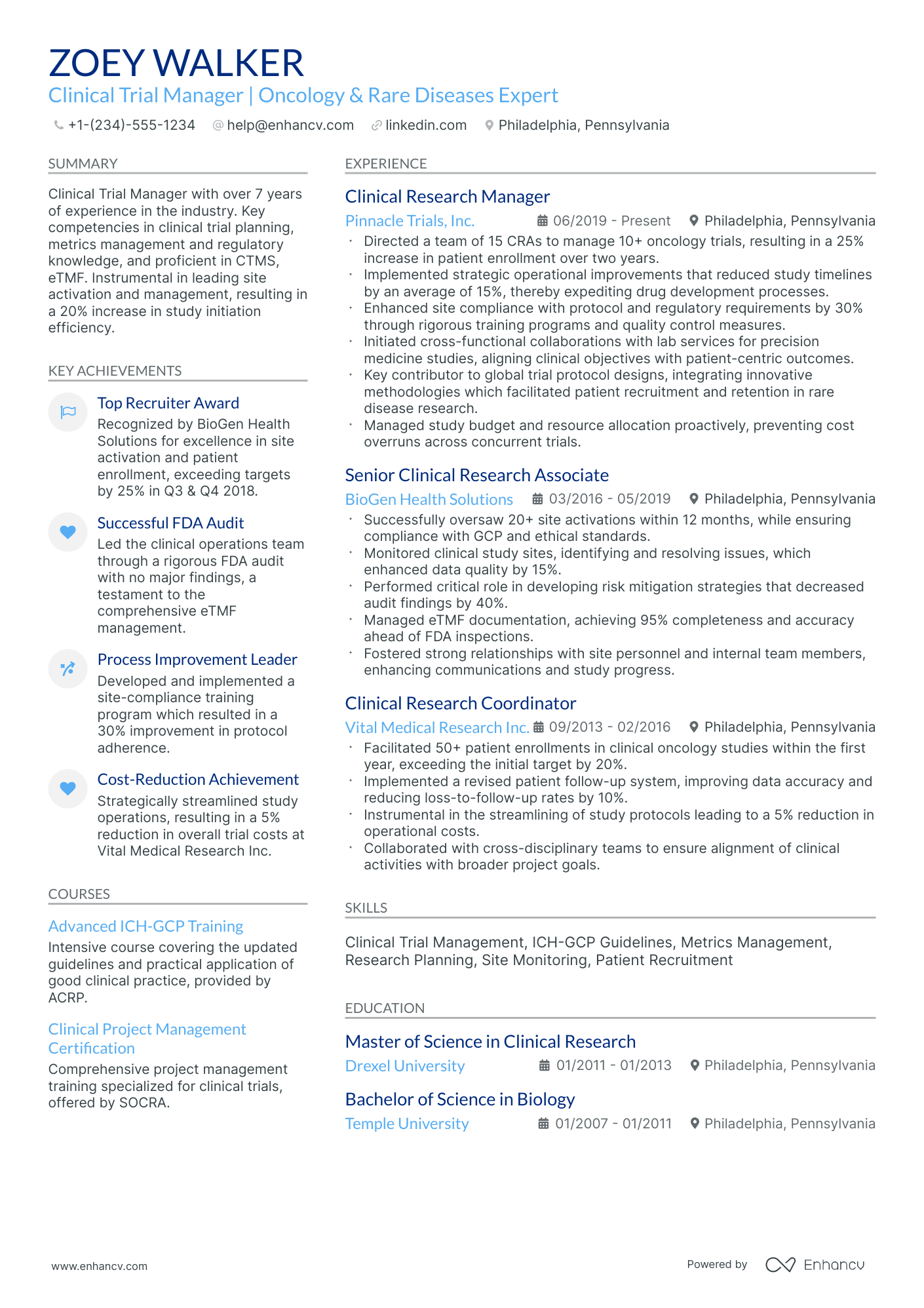
As a clinical trial manager, you may struggle to succinctly showcase the complexity of your project management skills and the depth of your regulatory knowledge on your resume. Our guide provides tailored strategies and industry-specific examples to help you effectively highlight your expertise and achievements, ensuring your resume stands out to potential employers.
- Clinical trial manager resumes that are tailored to the role are more likely to catch recruiters' attention.
- Most sought-out clinical trial manager skills that should make your resume.
- Styling the layout of your professional resume: take a page from clinical trial manager resume examples.
How to write about your clinical trial manager achievements in various resume sections (e.g. summary, experience, and education).
- Clinical Nurse Resume Example
- Ob Nurse Resume Example
- Assistant Nurse Resume Example
- Speech Pathologist Resume Example
- Veterinary Technician Resume Example
- Hospital Volunteer Resume Example
- Dermatology Medical Assistant Resume Example
- Life Coach Resume Example
- Ob Gyn Medical Assistant Resume Example
- Social Worker Resume Example
Don't stress out over your clinical trial manager resume format
Remember, the elaborate design of your clinical trial manager resume isn't what impresses recruiters most. They are primarily searching for candidates who meet the job requirements. The main aim of your resume should be to clearly and concisely explain why employers should hire you.
Here are four straightforward steps to consider in your clinical trial manager resume design:
- Organize your resume based on experience: Start with your most recent roles. Besides using reverse chronological order, choose jobs relevant to the position you're applying for.
- Include your contact details (and portfolio or LinkedIn link) in your resume's header to ensure recruiters can easily reach you. If considering adding a professional photo, check acceptable practices in different countries first.
- Don't omit essential clinical trial manager resume sections such as the summary or objective, experience, and education. These sections should reflect your career progression and align with job requirements.
- Maintain conciseness in your resume. For those with less than ten years of experience, a one-page format is advisable.
Regarding the format to submit your clinical trial manager resume, PDF is preferable. PDFs are more likely to maintain their formatting when processed through recruitment software or ATS, saving you time in the application process.
When selecting a font for your clinical trial manager resume, consider the following:
- Choose ATS-friendly fonts such as Exo 2, Volkhov, Lato, etc., to keep your resume's content legible;
- All serif and sans-serif fonts are easily readable by ATS;
- While Arial and Times New Roman are common choices, opting for unique typography can help your resume stand out.
Concerned about ATS compatibility with charts and infographics? Our recent study has debunked this and other myths.
Upload & Check Your Resume
Drop your resume here or choose a file. PDF & DOCX only. Max 2MB file size.
PRO TIP
The more trusted the organization you've attained your certificate (or degree) from, the more credible your skill set would be.
Clinical trial manager resume sections to answer recruiters' checklists:
- Header to help recruiters quickly allocate your contact details and have a glimpse over your most recent portfolio of work
- Summary or objective to provide an overview of your career highlights, dreams, and goals
- Experience to align with job requirements and showcase your measurable impact and accomplishments
- Skills section/-s to pinpoint your full breadth of expertise and talents as a candidate for the clinical trial manager role
- Education and certifications sections to potentially fill in any gaps in your experience and show your commitment to the industry
What recruiters want to see on your resume:
- Experience with clinical trial management systems (CTMS) and electronic data capture (EDC) tools.
- Understanding of Good Clinical Practice (GCP), FDA regulations, and ICH guidelines.
- Proven track record in leading and overseeing clinical trials from initiation to closure.
- Strong project management skills, with the ability to manage timelines, resources, and budgets effectively.
- Demonstrated ability to coordinate cross-functional teams and communicate with stakeholders at all levels, including investigators, IRBs, and regulatory authorities.
What to include in the experience section of your clinical trial manager resume
The resume experience section is perhaps the most important element in your application as it needs to showcase how your current profile matches the job.
While it may take some time to perfect your clinical trial manager experience section, here are five tips to keep in mind when writing yours:
- Assess the advert to make a list of key requirements and look back on how each of your past jobs answers those;
- Don't just showcase you know a particular skill, instead, you need proof in the form of tangible results (e.g. numbers, percent, etc.);
- It's perfectly fine to leave off experience items that don't bring anything extra to your skill set or application;
- Recruiters want to understand what the particular value is of working with you, so instead of solely featuring technologies, think about including at least one bullet that's focused on your soft skills;
- Take care with wording each bullet to demonstrate what you've achieved, using a particular skill, and an action verb.
The below clinical trial manager resume examples can help guide you to curate your professional experience, following industry-leading tips and advice.
- Led the coordination of a phase III global clinical trial for a leading oncology drug, overseeing cross-functional teams across 12 countries and enhancing patient enrollment by 40%.
- Managed a trial budget of $5M, optimizing resource allocation and reducing overhead costs by 15% without compromising on trial integrity or timelines.
- Implemented a risk-based monitoring approach, which increased data quality and efficiency, decreasing the frequency of onsite visits by 30% and the associated costs accordingly.
- Supervised early-stage clinical trials, focusing on novel immunotherapies and delivering results that expedited the transition from phase I to phase II within 18 months.
- Introduced new electronic data capture systems that reduced errors in trial data by 25% and improved the data analysis process.
- Forged strong relationships with investigative sites, increasing the number of high-performing sites by 20% and ensuring regular compliance with study protocols.
- Handle the end-to-end management of several phase II trials, which have led to the successful submission of two New Drug Applications within the last three years.
- Drove the adoption of patient-centric trial designs, which improved participant adherence by 35% and overall satisfaction rates among trial subjects.
- Cultivated a high-performing team environment which improved cross-departmental collaboration and reduced the average study close-out time by 25 days.
- Orchestrated a global phase III trial with a strategic focus on cardiovascular disease, directly contributing to the enrolment of over 5,000 study participants.
- Successfully managed vendor relationships leading to a 20% improvement in service delivery and a reduction in trial material waste by 10%.
- Ensured comprehensive protocol adherence and regulatory compliance, resulting in a 100% audit pass rate and positive feedback from regulatory agencies.
- Executed multiple phase II trials concurrently, while maintaining strict oversight on quality assurance measures and delivering study outcomes ahead of schedule.
- Negotiated contracts and service agreements with CROs and third-party vendors that led to a 12% reduction in costs across trial operations.
- Championed the development and implementation of a participant retention program that cut dropout rates by half.
- Streamlined the clinical trial management process for complex neurology studies, improving trial efficiency by 20% through the use of advanced project management software.
- Developed and maintained an integrated project plan that tracked progress against key milestones and communicated the status to all stakeholders regularly.
- Guided a team of CRAs, implementing ongoing training that boosted team performance metrics by 15%.
- Oversaw the expansion of a phase IV observational study which included real-world evidence, ultimately influencing insurance coverage decisions for a new rheumatoid arthritis treatment.
- Introduced innovative patient engagement strategies that enhanced participant retention to an exceptional 95% for the duration of the trial.
- Masterminded a standardized process for site performance reviews, boosting efficiency and highlighting areas for improvement that significantly enhanced site output.
- Managed clinical trial logistics for orphan disease medication trials, ensuring timely and cost-effective delivery of trial materials to over 100 sites globally.
- Designed and executed a comprehensive site selection process that identified and secured high-quality research sites exceeding performance expectations by 18%.
- Oversaw cross-functional teams and streamlined communication, resulting in advanced team cohesion and a 20% increase in problem-solving efficiency.
- Coordinated phase III pediatric vaccine trials which directly contributed to the successful FDA licensure of a new vaccine within the company's product portfolio.
- Implemented a continuous improvement initiative that identified a 15% increase in trial data accuracy and reduced data lock timelines by 10 days.
- Collaborated with regulatory bodies to ensure compliance with evolving industry standards, successfully navigating two major regulatory inspections without findings.
- Drove the execution and completion of a landmark phase II oncology trial that significantly shortened the typical duration from start to finish by 6 months.
- Instrumental in the establishment of a centralized monitoring hub that increased the efficiency of site monitoring by 22% and minimized travel expenditures.
- Spearheaded a successful investigator meeting series that bolstered site engagement and protocol comprehension, leading to higher quality data capture.
The following content includes information from "O*NET OnLine" by the U.S. Department of Labor, Employment and Training Administration (USDOL/ETA). Used under the CC BY 4.0 license. The data represents the top responsibilities present on the task lists for clinical trial manager professionals.
Top Responsibilities for Clinical Trial Manager:
- Schedule subjects for appointments, procedures, or inpatient stays as required by study protocols.
- Perform specific protocol procedures such as interviewing subjects, taking vital signs, and performing electrocardiograms.
- Assess eligibility of potential subjects through methods such as screening interviews, reviews of medical records, or discussions with physicians and nurses.
- Prepare study-related documentation, such as protocol worksheets, procedural manuals, adverse event reports, institutional review board documents, or progress reports.
- Inform patients or caregivers about study aspects and outcomes to be expected.
- Record adverse event and side effect data and confer with investigators regarding the reporting of events to oversight agencies.
- Monitor study activities to ensure compliance with protocols and with all relevant local, federal, and state regulatory and institutional polices.
- Oversee subject enrollment to ensure that informed consent is properly obtained and documented.
- Maintain required records of study activity including case report forms, drug dispensation records, or regulatory forms.
- Identify protocol problems, inform investigators of problems, or assist in problem resolution efforts, such as protocol revisions.
Quantifying impact on your resume
- Include the number of clinical trials managed to demonstrate the extent of practical experience and project management skills.
- List the size of patient cohorts managed in clinical trials to showcase the capacity to handle large-scale studies.
- Specify the budget amounts overseen to highlight financial responsibility and resource allocation proficiency.
- Mention the percentage of trials completed on time to emphasize efficiency and time management success.
- Quantify the number of sites you've coordinated across to show organizational skills and the ability to manage distributed teams.
- Detail the number of regulatory submissions completed to indicate experience with compliance and legal requirements.
- Document the improvement in patient recruitment numbers to display effectiveness in strategic planning and execution.
- Record the reduction in study duration or costs achieved to demonstrate an aptitude for process optimization and cost-saving measures.
Action verbs for your clinical trial manager resume
What to do if you don't have any experience
It's quite often that candidates without relevant work experience apply for a more entry-level role - and they end up getting hired.
Candidate resumes without experience have these four elements in common:
- Instead of listing their experience in reverse-chronological format (starting with the latest), they've selected a functional-skill-based format. In that way, clinical trial manager resumes become more focused on strengths and skills
- Transferrable skills - or ones obtained thanks to work and life experience - have become the core of the resume
- Within the objective, you'd find career achievements, the reason behind the application, and the unique value the candidate brings about to the specific role
- Candidate skills are selected to cover basic requirements, but also show any niche expertise.
Recommended reads:
PRO TIP
If the certificate you've obtained is especially vital for the industry or company, include it as part of your name within the resume headline.
Key hard skills and soft skills for your clinical trial manager resume
At the top of any recruiter clinical trial manager checklist, you'd discover a list of technical competencies, balanced with personal skills.
Hard or technical skills are your opportunity to show how you meet the essential responsibilities of the role. The ability to use a particular job-crucial technology or software would also hint to recruiters whether you'd need a prolonged period of on-the-job training - or you'd fit right in the job.
But to land your dream role, you'd also need to demonstrate a variety of soft or people resume skills . Employers care about soft skills as they show how each candidate would fit into the team and company culture.
Both types of skills are specific and to best curate them on your resume, you'd need to:
- Create a skill section within which you showcase your hard and soft skills and present how they help you succeed.
- List specific examples of projects, tasks, or competitions, within which your skill set has assisted your results.
- Soft skills are harder to measure, so think about situations in which they've helped you thrive. Describe those situations concisely, focusing on how the outcome has helped you grow as a professional.
- Metrics of success - like positive ROI or optimized workplace processes - are the best way to prove your technical and people skills.
Take a look at some of clinical trial manager industry leaders' favorite hard skills and soft skills, as listed on their resumes.
Top skills for your clinical trial manager resume:
Clinical Trial Management Systems (CTMS)
Electronic Data Capture (EDC) systems
Regulatory compliance (FDA, ICH, GCP)
Statistical analysis software (SAS, R)
Project management tools (MS Project, Trello)
Budgeting and financial management
Risk management methodologies
Data management and analysis
Clinical trial protocol development
Site management and monitoring tools
Leadership
Communication
Problem-solving
Team collaboration
Time management
Attention to detail
Adaptability
Conflict resolution
Critical thinking
Decision-making
Next, you will find information on the top technologies for clinical trial manager professonals from "O*NET OnLine" by the U.S. Department of Labor, Employment and Training Administration (USDOL/ETA). Used under the CC BY 4.0 license.
Top technologies for Clinical Trial Manager’s resume:
- Invivo Data EPX ePRO Management System
- PPD Patient Profiles
- Google Meet
- IBM SPSS Statistics
- The MathWorks MATLAB
PRO TIP
If you happen to have plenty of certificates, select the ones that are most applicable and sought-after across the industry. Organize them by relevance to the role you're applying for.
Certifications and education: in-demand sections for your clinical trial manager resume
Your academic background in the form of certifications on your resume and your higher degree education is important to your application.
The certifications and education sections pinpoint a variety of hard and soft skills you possess, as well as your dedication to the industry.
Add relevant certificates to your clinical trial manager resume by:
- Add special achievements or recognitions you've received during your education or certification, only if they're really noteworthy and/or applicable to the role
- Be concise - don't list every and any certificate you've obtained through your career, but instead, select the ones that would be most impressive to the role
- Include the name of the certificate or degree, institution, graduation dates, and certificate license numbers (if possible)
- Organize your education in reverse chronological format, starting with the latest degree you have that's most applicable for the role
Think of the education and certification sections as the further credibility your clinical trial manager resume needs to pinpoint your success.
Now, if you're stuck on these resume sections, we've curated a list of the most popular technical certificates across the industry.
Have a look, below:
The top 5 certifications for your clinical trial manager resume:
- Certified Clinical Research Professional (CCRP) - Society of Clinical Research Associates (SOCRA)
- Certified Clinical Research Associate (CCRA) - Association of Clinical Research Professionals (ACRP)
- Certified Clinical Research Coordinator (CCRC) - Association of Clinical Research Professionals (ACRP)
- Project Management Professional (PMP) - Project Management Institute (PMI)
- Regulatory Affairs Certification (RAC) - Regulatory Affairs Professionals Society (RAPS)
The content below includes information from "O*NET OnLine" by the U.S. Department of Labor, Employment and Training Administration (USDOL/ETA). Used under the CC BY 4.0 license. The data represents the top associations for clinical trial manager professionals.
Top US associations for a Clinical Trial Manager professional
- American Association for the Advancement of Science
- Association of Clinical Research Professionals
- Drug Information Association
- Professional Science Master's
- Society of Clinical Research Associates
PRO TIP
If you happen to have some basic certificates, don't invest too much of your clinical trial manager resume real estate in them. Instead, list them within the skills section or as part of your relevant experience. This way you'd ensure you meet all job requirements while dedicating your certificates to only the most in-demand certification across the industry.
Recommended reads:
Adding a summary or objective to your clinical trial manager resume
One of the most crucial elements of your professional presentation is your resume's top one-third. This most often includes:
- Either a resume summary - your career highlights at a glance. Select the summary if you have plenty of relevant experience (and achievements), you'd like recruiters to remember about your application.
- Or, a resume objective - to showcase your determination for growth. The perfect choice for candidates with less experience, who are looking to grow their career in the field.
If you want to go above and beyond with your clinical trial manager resume summary or resume objective, make sure to answer precisely why recruiters need to hire you. What is the additional value you'd provide to the company or organization? Now here are examples from real-life clinical trial manager professionals, whose resumes have helped them land their dream jobs:
Resume summaries for a clinical trial manager job
- With over a decade of dedicated experience in managing multi-phase clinical trials for a leading pharmaceutical company, I bring a profound understanding of trial protocols, patient recruitment strategies, and regulatory compliance. Key achievements include successfully leading a team through a complex phase 3 trial that resulted in FDA approval of a groundbreaking therapy.
- As an accomplished project manager in the construction industry for over 8 years, I am eager to leverage my expertise in project execution, stakeholder coordination, and risk management to transition into clinical trial management. My adeptness at ensuring project milestones and objectives positions me well for overseeing clinical study timelines and deliverables.
- Having directed research programs in academia for 15 years, my transition into clinical trial management comes with a robust background in scientific investigation, cross-disciplinary collaboration, and ethical research oversight. My successful funding acquisition and publication record underscores my ability to drive complex projects to fruition.
- Recently graduated with a Master's in Clinical Research, my enthusiasm for impacting patient care through rigorous clinical study is matched by strong analytical skills and a comprehensive understanding of GCP guidelines. While new to trial management, my academic excellence includes a thesis on optimizing clinical trial design.
- Seeking to apply my fresh perspective and keen interest in medical advancements, I aspire to contribute to clinical trials that enhance global health outcomes. With no professional experience yet, my intensive coursework, and volunteer work in patient care provide a solid foundation for understanding the demands and responsibilities of clinical studies.
- Bringing forth a rich background in biomedical research and a Ph.D. in molecular biology, I am prepared to embark on a career in clinical trial management. My scientific acumen, coupled with a 4-year engagement in managing collaborative research projects, has equipped me with the critical thinking and organizational skills vital for overseeing clinical trials.
Average salary info by state in the US for Clinical Trial Manager professionals
Local salary info for Clinical Trial Manager.” Source: My Next Move, National Center for O*NET Development. Accessed 10/15/2024
| State | Average Salary (in USD) |
|---|---|
| US National Average | $157,740 |
| California (CA) | $198,580 |
| Texas (TX) | $109,300 |
| Florida (FL) | $92,490 |
| New York (NY) | $142,150 |
| Pennsylvania (PA) | $126,870 |
| Illinois (IL) | $138,340 |
| Ohio (OH) | $130,230 |
| Georgia (GA) | $108,480 |
| North Carolina (NC) | $163,910 |
| Michigan (MI) | $137,660 |
Bonus sections for your clinical trial manager resume
Looking to show more personality on your clinical trial manager resume? Then consider including a couple of extra sections.
They'd benefit your application by highlighting your most prominent:
Key takeaways
- Pay special attention to the tiny details that make up your clinical trial manager resume formatting: the more tailored your application to the role is, the better your chances at success would be;
- Select the sections you include (summary or objective, etc.) and formatting (reverse-chronological, hybrid, etc.) based on your experience level;
- Select experience items and, consequently, achievements that showcase you in the best light and are relevant to the job;
- Your profile will be assessed both based on your technical capabilities and personality skills - curate those through your resume;
- Certifications and education showcase your dedication to the particular industry.